Xoran Technologies Submits FDA 510(k) Application for TRON™

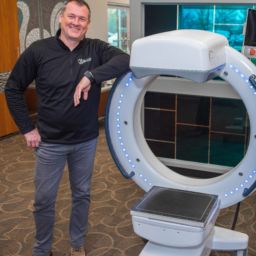
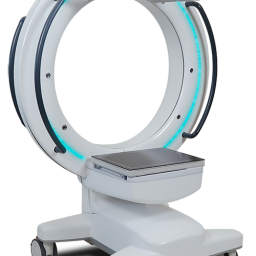
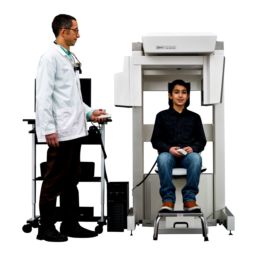
ANN ARBOR, MI, June 27, 2022–Xoran Technologies announces its recent submission of an FDA 510(k) application for TRONTM—a mobile, open-bore fluoroscopy cone beam computed tomography (CT) for full-body point-of-care imaging.
“The TRON device submission is a huge step for Xoran in terms of expanding our imaging capability overall,” says Misha Rakic, Xoran CEO. “With this thru-bore CT that is truly mobile, compact, and able to perform low dose imaging, we’re expanding our existing competence out of the realm of ‘head only’ CT and progressing to imaging the entire body.”
This month Xoran also announced that it had begun work on Phase 2 of its mobile lung grant—the goal of which is to confirm the safety and utility of a future thoracic point-of-care CT system in support of an FDA submission. These research and development efforts for lung CT are supported by a recent grant award from the National Heart, Lung, and Blood Institute (NHLBI) through the National Institutes of Health (NIH).
The compact, mobile design and engineering of TRON for medical imaging marks a milestone toward democratizing access to diagnostic imaging.
About Xoran Technologies
Since 2001, Xoran is the pioneer and medical market leader in low-dose radiation, cone beam CT systems specifically designed for the patient’s point-of-care. Providers around the world rely on our industry leading MiniCAT™, xCAT™, and veterinary CT systems: VetCAT and vTRON, to diagnose and treat patients.
Xoran is based in Ann Arbor, Michigan.
For more information visit www.xorantech.com/
© 2022 Xoran Technologies, LLC
Media Contact
Aramide Boatswain
+1.734-709-0464
info@xorantech.com